www.medical-devices.tech
07
'24
Written on Modified on
Siemens Healthineers News
TRUEBEAM & EDGE RADIOTHERAPY SYSTEMS WITH HYPERSIGHT IMAGING CLEARED BY FDA
Varian, a Siemens Healthineers company, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for TrueBeam and Edge radiotherapy systems featuring HyperSight imaging solution.
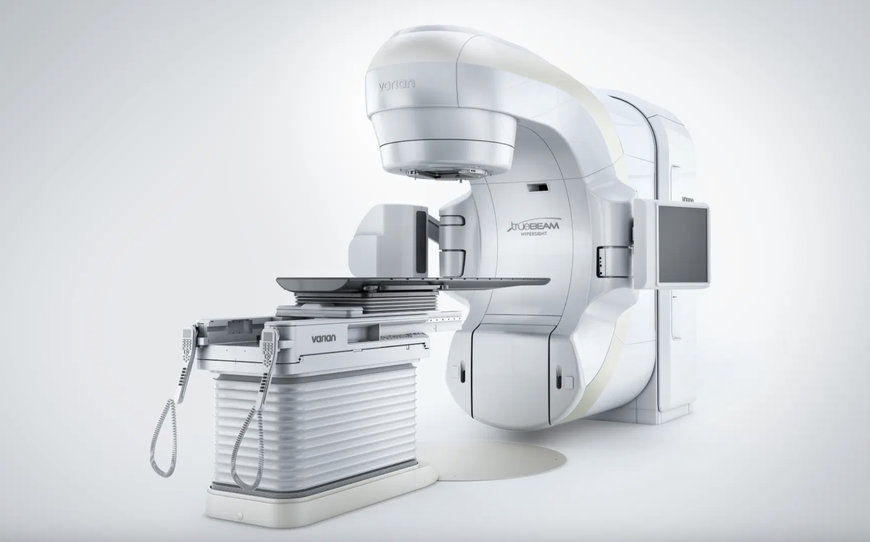
By extending the groundbreaking HyperSight imaging solution optional feature to the TrueBeam and Edge systems, Varian continues to revolutionize imaging inside the radiotherapy treatment room and adds new capabilities and workflows across the entire suite of linear accelerators. HyperSight empowers clinicians to accurately tailor treatments to each individual patient with the goal of improving patient outcomes.
HyperSight imaging allows clinicians to acquire high-quality images during a patient's daily course of radiation treatments. The enhanced image quality is designed to improve the ability to target tumor volumes more precisely and spare healthy tissue for patients receiving radiation therapy treatments. With the addition of HyperSight, linear accelerators across the Varian portfolio can now produce images that deliver the Hounsfield Unit (HU) accuracy necessary for treatment planning directly on the acquired conebeam CT (CBCT) images. As a result, this technology can be used for adaptive planning to adjust for anatomical changes to the tumour and surrounding organs over the course of treatment without the need for an additional trip to a separate CT scanner.
HyperSight on TrueBeam and Edge acquires images for all anatomical sites with a 50% faster gantry rotation, significantly reducing acquisition time. In contrast, traditional CBCT scans can take up to 60 seconds, depending on the anatomical site being scanned. A reduction in CBCT acquisition time reduces motion-related artifacts due to patient movement. A shorter image acquisition time may minimize patient discomfort and anxiety due to less time on the treatment table.
Initially launched in 2022 on Varian’s Ethos and Halcyon therapy systems, HyperSight has been gaining popularity among clinicians and researchers alike. With nine studies either conducted, in progress, or to be initiated across eight institutions, Varian is committed to better understanding the role HyperSight may play in advancing care and enhancing the patient experience. The customer-led Intelligent Imaging Consortium, created by Varian, offers HyperSight users a forum to collaborate and innovate on trials and studies.
www.siemens-healthineers.com


