www.medical-devices.tech
27
'23
Written on Modified on
Abbott News
ABBOTT PRESENTS LATE-BREAKING DATA ON ESPRIT™ BTK DRUG-ELUTING RESORBABLE SCAFFOLD
Abbott's new investigational drug-eluting Esprit BTK (below-the-knee) resorbable scaffold is made of naturally dissolving material that disappears over time after it's opened a clogged artery.
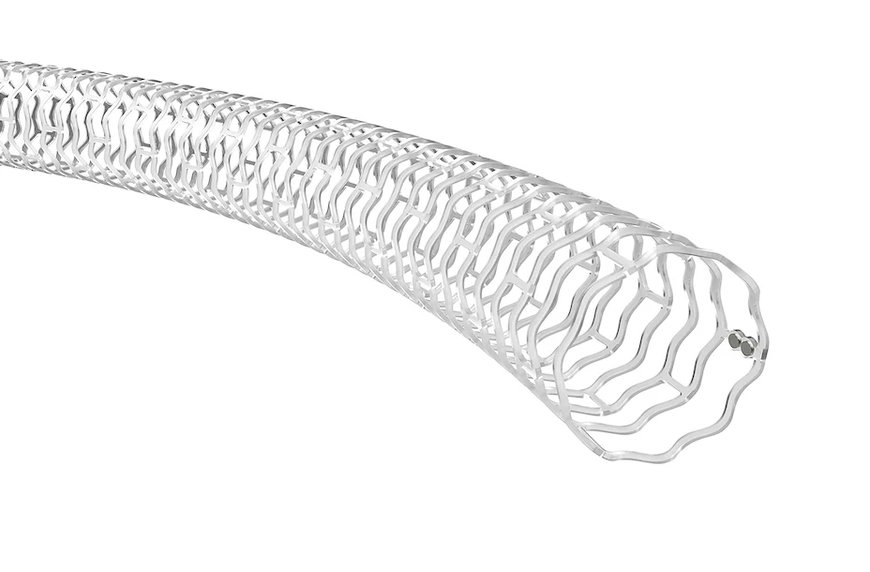
Abbott announced late-breaking data from the LIFE-BTK clinical trial evaluating the Esprit™ BTK Everolimus Eluting Resorbable Scaffold System (Esprit BTK) in people with chronic limb-threatening ischemia (CLTI). CLTI is a severe stage of peripheral artery disease (PAD) due to advanced arterial blockage in the lower extremities. The LIFE-BTK trial met both of its primary safety and effectiveness endpoints, demonstrating that Esprit BTK reduces disease progression and helps improve medical outcomes compared to the current standard of care, balloon angioplasty.
Data from LIFE-BTK was presented as a late-breaking clinical trial at the 35th Transcatheter Cardiovascular Therapeutics (TCT) Conference in San Francisco and simultaneously published in the New England Journal of Medicine.
More than 200 million people worldwide have PAD with nearly 11% affected by CLTI, a severe form of PAD. In these people, blocked vessels impair blood flow to the lower extremities, often leading to severe pain, non-healing wounds, and, in some cases, the need for limb amputation. Balloon angioplasty, a procedure in which a small balloon is inserted into the artery to open the blockage, is currently one of the approved procedures for people in the U.S. with CLTI. However, in many instances, the vessels become blocked again over time following balloon angioplasty, requiring additional treatment.
Abbott's Esprit BTK is a drug-eluting resorbable scaffold comprised of materials similar to dissolving sutures. Unlike metal stents, Esprit BTK is not a permanent implant, as blocked vessels only need support for a few months after the blockage is cleared. At that point, the vessel can stay open on its own, which is why Esprit BTK is designed to serve a temporary, yet crucial role.
The LIFE-BTK trial enrolled 261 people worldwide. The study aimed to investigate whether Esprit BTK could offer greater benefits than the current option of balloon angioplasty to open blocked arteries in the leg and to keep the arteries open. The trial's primary efficacy endpoint evaluated primary patency plus limb salvage (the ability of the vessel to stay open, and increasing the time until another intervention is needed). The trial met the efficacy endpoint and showed that Esprit BTK is superior to balloon angioplasty in lowering the risk of total obstruction of the target vessel, narrowing of the target lesion, major amputation and repeat interventions of the target lesion. The primary safety endpoint evaluating freedom from above-the-ankle amputation, major reintervention at six months and death within 30 days showed that Esprit BTK was non-inferior (similar performance) to balloon angioplasty.
Results of the one-year LIFE-BTK clinical trial showed:
- Esprit BTK had significantly greater freedom from the primary efficacy clinical events of 74.5 vs 43.7% (balloon angioplasty) for people with CLTI.
- The trial's powered secondary endpoints revealed that Esprit BTK was superior to balloon angioplasty at reducing vessel re-narrowing (25.8% improvement) and sustaining the openness of the vessels (14.2% improvement).
Based on the strength of the LIFE-BTK trial results, Abbott intends to submit the Esprit BTK Everolimus Eluting Resorbable Scaffold System for review by the U.S. Food and Drug Administration (FDA).


