www.medical-devices.tech
24
'23
Written on Modified on
Abbott News
ABBOTT RECEIVES FDA APPROVAL FOR TACTIFLEX ABLATION CATHETER
TactiFlex Ablation Catheter, Sensor Enabled, is the world's first ablation catheter designed with a unique flexible electrode tip and contact force sensing to treat patients with atrial fibrillation.
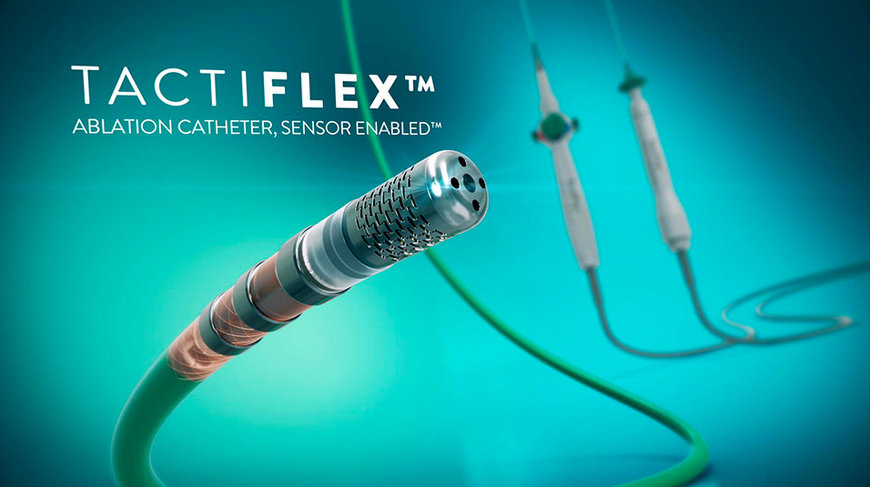
Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the company's TactiFlex™ Ablation Catheter, Sensor Enabled™, the world's first ablation catheter with a flexible tip and contact force technology.
Used to perform an ablation procedure to treat atrial fibrillation (AFib), the most common abnormal heart rhythm, the TactiFlex catheter can result in reduced procedure times and better safety when compared to the company's previous generation catheters.
More than 37 million people worldwide live with AFib and numbers are predicted to more than double by 2050. An additional five million cases are diagnosed every year, indicating a growing health challenge that demands innovative solutions for patients and their physicians.
New Catheter Pairs with Abbott's Other Best-in-Class Solutions for AFib Patients
The TactiFlex catheter is designed to be used with Abbott's EnSite X EP System, an industry-leading heart mapping system, which allows physicians to view and precisely identify areas in the heart that require ablation.
Unlike other catheters on the market, the TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall. This helps direct fluid to the treated tissue1 and allows for more accurate positioning of the catheter – providing up to two times higher stability in a beating heart – for consistent therapy delivery.
The Abbott TactiFlex catheter generated strong clinical outcomes in the TactiFlex AF IDE study. The study showed the catheter created fast, safe lesions to treat AFib with over 99% acute procedural success.
The TactiFlex catheter is also approved for use in Europe, Japan, Africa and Australia.
www.abbott.com


