www.medical-devices.tech
19
'23
Written on Modified on
Abbott News
TRICLIP DEVICE PROVES BENEFICIAL FOR PEOPLE WITH TRICUSPID VALVE DISEASE
Data presented at EuroPCR 2023 build on prior study results demonstrating Abbott's TriClip is a safe and effective therapy to treat leaky tricuspid valves.
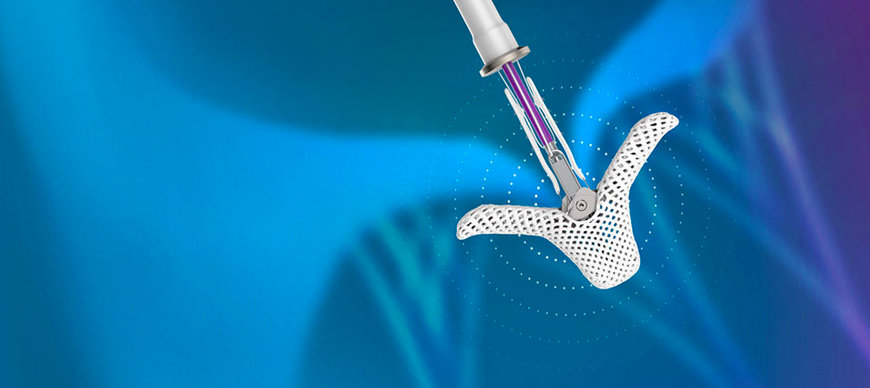
Abbott announced late-breaking data that add to the body of clinical evidence supporting the benefits of the TriClip™ transcatheter edge-to-edge repair (TEER) system in treating patients with leaky tricuspid valves. Abbott's TriClip device is a first-of-its-kind, minimally invasive device designed specifically for tricuspid heart valve repair.
The results were presented at EuroPCR, the annual meeting of the European Association of the Percutaneous Cardiovascular Interventions, held in Paris from May 16-19, 2023.
30-Day Outcomes From the TriClip bRIGHT Study
The results of the bRIGHT study, the largest real-world dataset for transcatheter tricuspid valve repair, support the safety and effectiveness of the TriClip system in patients with tricuspid regurgitation (TR). Outcomes from 511 patients in 26 sites across Europe for this prospective, multi-center, single-arm post-market study were presented for the first time at EuroPCR 2023.
Key findings through 30 days include:
- Significant TR reduction. Repair with the TriClip system resulted in reduction of TR grade to moderate or less for 77% of the patients.
- Significant clinical and quality of life improvements. 79% of participants achieved New York Heart Association (NYHA) Functional Class I/II (meaning they reached a point of slight or no limitation of physical activity), a nearly 60% improvement from the baseline proportion of 20%. In addition, more than half (56%) of patients reported a 15-point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score (a self-assessment of symptoms, physical and social limitations, and quality of life), representing a substantial improvement in quality of life and health status.
- A strong safety profile. Only 2.5% of patients who received the device experienced a major adverse event, a composite of cardiovascular death, heart attack, stroke, new onset of kidney failure and surgery for device-related adverse events.
TriClip is approved for use in more than 50 countries, including in Europe and Canada, and is an investigational device in the U.S.


