www.medical-devices.tech
23
'23
Written on Modified on
Abbott News
ABBOTT provides new data monitoring equipment for heart failure patients
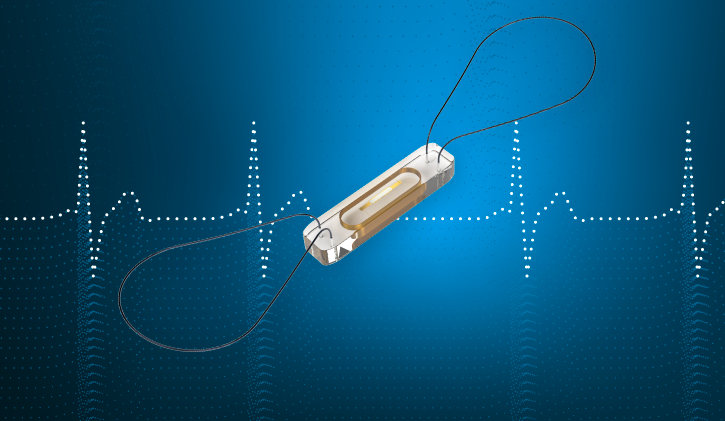
Abbott introduces the CardioMEMSTM HF System, a small implanted sensor that can remotely detect signals of worsening heart failure.
The analysis is the first to give doctors specific insight into how remotely monitoring patients with technology like CardioMEMS can provide an early warning against worsening heart failure and significantly reduce mortality risk by 25% at two years in HFrEF patients.
The meta-analysis of three randomized, controlled trials (CHAMPION, GUIDE-HF and LAPTOP-HF) was presented at the Technology and Heart Failure Therapeutics (THT) Conference in Boston, Mass. (March 20-22, 2023). The data reinforce that – in addition to providing an early warning system against worsening heart failure – remote monitoring technology like CardioMEMS can help doctors more proactively make changes to a patient’s treatment plan before the disease advances, often resulting in repeat hospitalizations.
A patient’s risk of mortality significantly rises2 with each heart failure-related hospitalization, making it critical that treatment plans aim to manage the disease and keep patients out of the hospital.
The CardioMEMS sensor is a paperclip-sized device that, once placed in the pulmonary artery during a minimally invasive procedure, monitors for pressure changes that indicate worsening heart failure. It wirelessly transmits daily readings to a patient’s clinical team – empowering the patient and their care team to manage their condition from virtually anywhere. HFrEF, the type of heart failure assessed within the latest meta-analysis, occurs when the heart muscle is weakened and not able to pump out enough blood for the body, representing roughly half3 of all cases of heart failure.
New Clinical Data Reveal Life-Extending Benefits of Remote Pressure Monitoring
Within the new analysis, data from the CHAMPION, GUIDE-HF and LAPTOP-HF4 trials were combined to assess the mortality and heart failure hospitalizations of 1,350 HFrEF patients. Among the three randomized trials, more than 650 patients were subjected to hemodynamic monitoring and 684 received the control. Heart failure hospitalizations were analyzed over a 12-month follow-up period and all-cause mortality was evaluated across 24 months.
The meta-analysis validated that hemodynamic monitoring can slow the progression of heart failure in HFrEF patients by significantly decreasing heart failure-related hospitalizations and improving survival.
The CardioMEMS HF System is approved for use in people living with New York Heart Association (NYHA) Class II or III heart failure who either have been hospitalized for heart failure in the previous year or for patients who undergo a blood test showing elevated levels of biomarkers known as natriuretic peptides, which indicate worsening heart failure. The hemodynamic data are used by physicians for heart failure management with the goal of controlling pulmonary artery pressures and reducing heart failure hospitalizations.
Indications and Safety Information for the CardioMEMS HF System visit: https://www.cardiovascular.abbott/us/.
www.abbott.com


